Chemical Reaction Engineering - Section 1 (2)
- Home
- Chemical Engineering
- Chemical Engineering Questions and Answers
- Chemical Reaction Engineering - Section 1
Chemical Reaction Engineering - Section 1
The MCQs related to Chemical Reaction Engineering
are available in this section and you can solve the problems to test your skills.
| 9. | Pick out the correct statement. |
|||||||
Answer: Option C Explanation: No answer description available for this question. Let us discuss. |
| 10. | For a solid catalysed chemical reaction, the effectiveness of solid catalyst depends upon the __________ adsorption. |
|||||||
Answer: Option B Explanation: No answer description available for this question. Let us discuss. |
| 11. | The excess energy of reactants in a chemical reaction required to dissociate into products is termed as the __________ energy. |
|||||||
Answer: Option A Explanation: No answer description available for this question. Let us discuss. |
| 12. | Radioactive decay follows __________ order kinetics. |
|||||||
Answer: Option A Explanation: No answer description available for this question. Let us discuss. |
| 13. | BET apparatus |
|||||||
Answer: Option D Explanation: No answer description available for this question. Let us discuss. |
| 14. | For a zero order chemical reaction, the |
|||||||
Answer: Option D Explanation: No answer description available for this question. Let us discuss. |
| 15. | For an isothermal variable volume batch reactor, the following relation is applicable for a first order irreversible reaction. |
|||||||
Answer: Option B Explanation: No answer description available for this question. Let us discuss. |
| 16. | For the irreversible elementary reactions in parallel viz |
|||||||
Answer: Option A Explanation: No answer description available for this question. Let us discuss. |

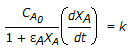
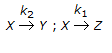 , the rate of disappearance of 'X' is equal
, the rate of disappearance of 'X' is equal